Oncogenic PI3K and K-Ras Drive De Novo Lipid Synthesis via mTORC1-Dependent SREBP Activation
This study investigated the role of oncogenic PI3K and K-Ras in stimulating de novo lipid synthesis. The researchers discovered that both oncogenes, frequently activated in cancer, are sufficient to induce lipid production through the common downstream activation of mTORC1.
**Experimental Approach:**
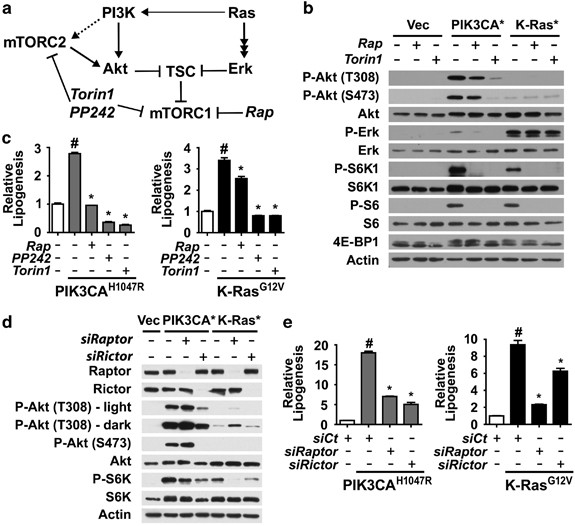
* Isogenic cell lines expressing either empty vector, oncogenic PIK3CA (PI3K), or K-Ras were generated in MCF10a cells, a non-transformed human breast epithelium cell line.
* Oncogene expression resulted in growth factor-independent activation of mTORC1, as evidenced by phosphorylation of downstream targets S6K1, 4E-BP1, and ribosomal S6.
* C-acetate labeling was used to assess de novo lipid synthesis, avoiding confounding effects of oncogenes on glucose uptake.
* Both oncogenic PI3K and K-Ras significantly increased acetate incorporation into lipids, an effect reduced by rapamycin and further decreased by mTOR kinase inhibitors PP242 and Torin1.
* siRNA knockdown of mTORC1 components (Raptor) or mTORC2 components (Rictor) revealed that mTORC1, but not mTORC2, was responsible for the oncogene-induced lipogenesis.
**Mechanism of Action:**
* The study explored the role of SREBP transcription factors, previously known to be stimulated by mTORC1 in lipid synthesis.
* Oncogenic PI3K and K-Ras increased both precursor and mature forms of SREBP1 and SREBP2, an effect blocked by rapamycin and Torin1.
* Increased levels of canonical SREBP target genes (ACC1, FASN, and SCD) were observed at both transcript and protein levels, with mTORC1 inhibitors reducing their expression.
* Knockdown of SREBP1 and SREBP2, particularly SREBP2, significantly inhibited oncogene-induced lipogenesis, indicating their critical role in the process.
* SCD, the enzyme responsible for generating monounsaturated fatty acids (MUFAs) prevalent in membrane phospholipids, was identified as a key target of SREBP activation.
**Relevance to Cancer:**
* A panel of eight genetically defined breast cancer cell lines with oncogenic activation of PI3K or Ras pathways exhibited constitutive mTORC1 signaling and de novo lipogenesis.
* Inhibition of mTOR reduced lipid synthesis and decreased expression of SREBP target genes in these cancer cell lines.
* Knockdown of SREBP isoforms, particularly SREBP2, significantly reduced proliferation, growth, and viability of the cancer cells.
* The study demonstrated a requirement for SREBP in the aberrant, growth factor-independent growth and proliferation of oncogene-expressing cells.
**Clinical Implications:**
* Analysis of breast cancer data from The Cancer Genome Atlas (TCGA) revealed a strong association between mTORC1 activation and increased expression of SREBP target genes, particularly FASN and SCD.
* This data suggests that the mTORC1-SREBP pathway plays a crucial role in breast cancer development and progression.
**Conclusion:**
This study provides compelling evidence that oncogenic PI3K and K-Ras drive de novo lipid synthesis through mTORC1-dependent activation of SREBP isoforms, particularly SREBP2. The aberrant activation of this pathway contributes to the growth and survival of breast cancer cells. These findings highlight the potential of targeting the mTORC1-SREBP pathway as a therapeutic strategy for breast cancer.